Drugs fail to slow memory and thinking decline in rare genetic form of Alzheimer’s
Published By Alzheimer's Research UK [English], Mon, Jun 21, 2021 9:20 AM
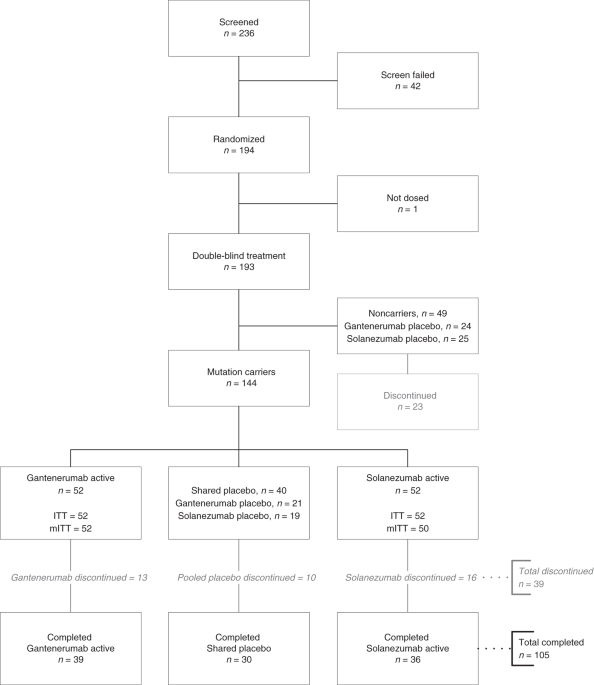
Today (Monday 21 June) the scientific publication Nature Medicine published findings from an international clinical trial (DIAN-TU) testing the potential Alzheimer’s drugs solanezumab and gantenerumab in people with a rare, inherited form of Alzheimer’s disease.
The results confirm top-line results originally presented in 2020, which showed the drugs failed to have a meaningful benefit on memory and thinking in people with familial Alzheimer’s disease (FAD).
It also shows additional data on how the drugs affected key brain changes associated with the disease.
Dr Susan Kohlhaas, Director of Research at Alzheimer’s Research UK:
“Investigating potential new drugs in people with rare-inherited forms of Alzheimer’s disease not only offers us unique insight into the development of familial Alzheimer’s but also the more common, non-genetic form of the disease. “While we have known for a while that gantenerumab and solanezumab weren’t able to slow down or reverse memory and thinking decline in this trial, the new data is further evidence that the drugs do target the biological processes in the brain they were designed to hit and have an impact on markers of neurodegeneration. “There are also signs that treating early in the disease process may be most beneficial. As this was a very small study one key aim will be to get more people with this rare form of dementia involved in the trial so that researchers can learn about more subtle cognitive changes that occur early in the disease. “We cannot thank the brave and committed volunteers who took part in this research enough, and we know as a field, we will have to redouble our efforts to help those with this particularly devastating form of Alzheimer’s. “Recently a drug called aducanumab was approved in the US for people with Alzheimer’s disease, but it is not yet clear whether it will be granted approval in this country and it is important that we work towards a range of approaches so that we can bring about effective treatments as soon as possible. “For more information about this trial, or dementia research, contact the Alzheimer’s Research UK Dementia Research Infoline on 0300 111 5111.”
The DIAN-TU – the Dominantly Inherited Alzheimer Network Trials Unit – is an international drug trial to see whether the rare genetic form of Alzheimer’s – familial Alzheimer’s disease (FAD) – can be slowed down by potential new medicines.
Scientists tested two potential drugs – solanezumab and gantenerumab – both targeting amyloid, the hallmark Alzheimer’s protein.
Participants in the trial were from around the world, including a centre at University College London in the UK. Volunteers either knew they have an Alzheimer’s disease-causing mutation or were unaware of their genetic status but had a FAD mutation in their family.
DIAN-TU is the first study of its kind, testing the use of antibody drugs to prevent or slow down the onset of Alzheimer’s in those who have a genetic risk of developing this disease early in life.
A build-up of amyloid around brain nerve cells is thought to start a cascade of processes leading to the death of nerve cells and eventually a loss of memory.
Solanezumab and gantenerumab target this protein. Both drugs have had a complicated history and both work in slightly different ways.
Solanezumab is designed to clear clumps of amyloid from the brain, and gantenerumab is designed to stop the large build-up of amyloid forming.
The way both drugs are given is also different. Solanezumab is given by infusion and gantenerumab was given as a monthly injection. The drugs were given in increasingly higher doses over a period of four years.
The study failed to show benefit to study volunteer’s memory and thinking.
In this study, 52 people received gantenerumab, which led to a reduction in the amount of amyloid in the brain. Gantenerumab also lowered the amount of tau protein in the brain as well as slowing the rise of a marker of disease – Neurofilament light chain (Nfl). Nfl is a structural component of nerve cells in the brain. It leaks from the brain when these nerve cells become damaged and can end up in the bloodstream and spinal fluid.
About one percent of people with Alzheimer’s disease will have the genetic form, known as familial Alzheimer’s disease (FAD).
It is genetically inherited or passed down through families. Individuals who have a parent with a genetic mutation have a 50 percent chance of carrying the gene and many experiences an onset of symptoms in their 30s and 40s.
A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease by Salloway et al., is published in Nature Medicine, on 21/06/2021
Press release distributed by Media Pigeon on behalf of Alzheimer's Research UK, on Jun 21, 2021. For more information subscribe and follow